
Excerpts:
I was reading here that there’s a new cholesterol-lowering drug in the pipeline. The Food and Drugs Administration in the US has approved the injectable agent mipomersen for the treatment of ‘homozygous familial hypercholesterolaemia – a genetic cause of raised cholesterol levels associated with cardiovascular disease developing early in life. Mipomersen has been shown to reduce low density lipoprotein (LDL) levels by about a quarter. The hope is that this will help reduce the risk of individuals suffering premature death from, most likely, heart attack.
I’ve used the word ‘hope’ in the preceding sentence but, in reality, the FDA panel that has ratified mipomersen has no idea whether it has health benefits or not. That’s because the studies used to assess this drug were not large enough in scope to detect change in ‘clinical endpoints’ such as risk of heart attack or death. In other words, the licensing of this drug has been on the basis of its impact on ‘surrogate endpoints’ (in this case, cholesterol levels), rather than clinical endpoints (the thing that really matters).
Read the full article and comment here: http://www.drbriffa.com/2012/11/02/another-new-cholesterol-lowering-drug-is-licensed-despite-no-evidence-of-benefits-for-health/




 HHS Secretary Kennedy Breaks His Promise: "War on Saturated Fat" Kept in Tact with New U.S. Dietary Guidelines
HHS Secretary Kennedy Breaks His Promise: "War on Saturated Fat" Kept in Tact with New U.S. Dietary Guidelines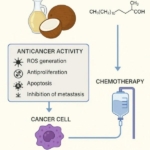 Research Continues to Show Virgin Coconut Oil's Effectiveness in Treating Cancer
Research Continues to Show Virgin Coconut Oil's Effectiveness in Treating Cancer Coconut Oil Continues to Benefit Alzheimer's Patients over Drugs as Studies Continue for Neurological Benefits
Coconut Oil Continues to Benefit Alzheimer's Patients over Drugs as Studies Continue for Neurological Benefits How the Simple High-Fat Low-Carb Ketogenic Diet Continues to Change People's Lives
How the Simple High-Fat Low-Carb Ketogenic Diet Continues to Change People's Lives New Studies Continue to Show that Coconut Oil is the Best Oil for Treating Skin Conditions and Maintaining Healthy Skin and Teeth
New Studies Continue to Show that Coconut Oil is the Best Oil for Treating Skin Conditions and Maintaining Healthy Skin and Teeth